Car T Therapy Canada
CAR T-Cell Therapy is now available at a select number of sites within Canada upon approval. CAR T-cell therapy is a type of immunotherapy meaning your underlying disease is treated by stimulating your immune response to your cancer cells.

Car T Cell Therapy Sino Biological
Kymriah and Yescarta are both used to treat aggressive blood cancers that have not responded to other.

. Canadian patient in landmark trial shares his story A CAR T-cell therapy clinical trial led by Dr. In Canada CAR T therapy is currently regulated in the same way as pharmaceuticals and there are currently two commercial CAR T therapies that have been. It was initially approved by Health Canada in 2018 for relapsedrefractory Diffuse Large B-Cell.
Other possible serious side effects of CAR T-cell therapy can include. Alberta is now the third province to offer the treatment. The cells are genetically engineered to recognize a patients own tumour and then returned to the patients.
Ad Brexucabtagene Autoleucel Download Patient Brochure And Read Medication Guide. Abnormal levels of minerals in the blood such as low potassium sodium or phosphorous. CAR T-cell therapy is a precision medicine treatment meaning treatment that is tailored to individual patients.
For the first time. Its a form of CAR-T immunotherapy in which a patients blood cells are removed reprogrammed to attack cancer and then re-injected back into their body. It also belongs to a new group of cancer treatments known as.
Natasha Kekre of The Ottawa Hospital and. Challenges for Implementation in Canada. The goal of this procedure is to cure.
Ad Brexucabtagene Autoleucel Download Patient Brochure And Read Medication Guide. How is CAR T cell therapy available to BC Pediatric Patients. Health Canada approved product Kymriah Novartis Currently not available 25.
We are seeing some very promising. Other serious side effects. For Clinical Trials.
Learn About An FDA-Approved Therapy Find Authorized Treatment Centers. Health Canada approved the first therapy in September 2018. Thursday October 24 2019 New CAR T-cell Clinical Trials in the United States Have Extended Enrollment to Canadian Myeloma Patients.
CAR T therapy starts by extracting a patients immune cells from their body. CAR T-cell therapy is called a living therapy That is because the CAR T-cells go on to multiply in the body and continue fighting the cancer. Two commercial CAR-T therapies have been approved for use in Canada.
Referrals must first be made within Canada out-of-province prior to exploring out-of-country. In 2017 autologous chimeric antigen receptor CAR T-cell therapy tisagenlecleucel Kymriah Novartis. There are several types of CAR T-cell therapy.
Eligibility and procedure Only people with certain kinds of blood cancers can. Its a labour-intensive process that involves taking blood from a patient and. CAR-T therapy is still considered a new and innovative therapy for the treatment of lymphoma.
Our goal is to build Canadian expertise and capacity for. Traveling to the US. November 9 2021 CAR T-cell therapy.
The new treatment is funded with 15 million from the government and the Alberta Cancer Foundation. Learn About An FDA-Approved Therapy Find Authorized Treatment Centers. Natasha Kekre is working with other hospitals across Canada to develop a made-in-Canada approach for CAR-T cancer therapy.
They may even continue to grow and work over. CAR T-cell therapy modifies the cells so they are able to identify the cancer cells and destroy them.

New Car T Cell Therapy Extends Remission In Heavily Relapsed Multiple Myeloma Patients Newsroom Ut Southwestern Dallas Texas
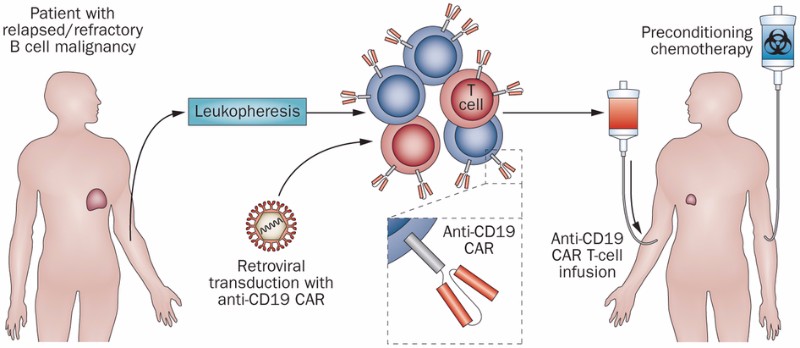
A Cure For Cancer How Car T Cell Therapy Is Revolutionizing Oncology
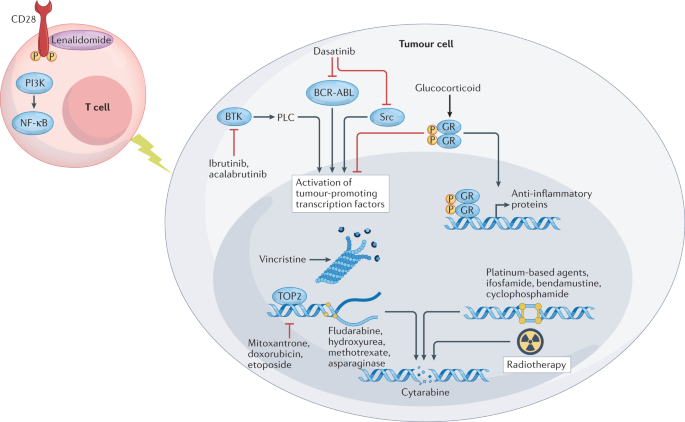
Preparing For Car T Cell Therapy Patient Selection Bridging Therapies And Lymphodepletion Nature Reviews Clinical Oncology
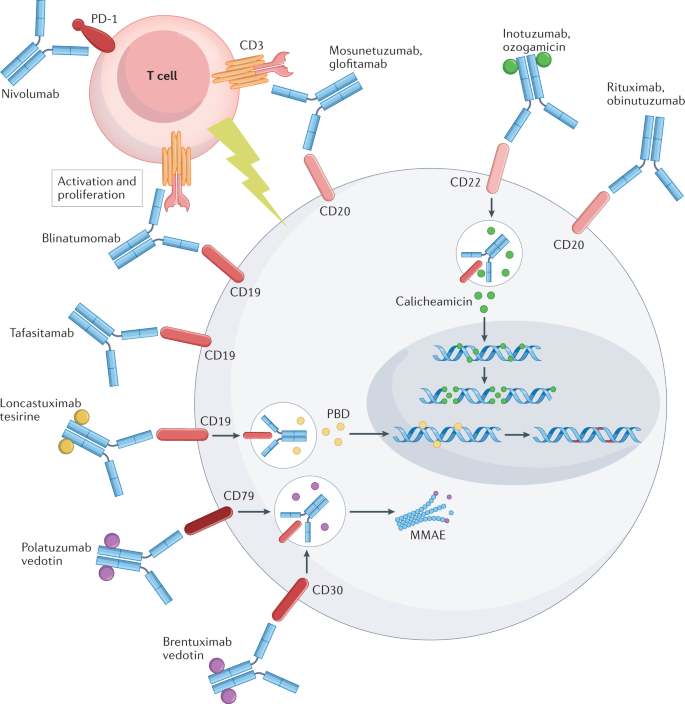
Preparing For Car T Cell Therapy Patient Selection Bridging Therapies And Lymphodepletion Nature Reviews Clinical Oncology
No comments for "Car T Therapy Canada"
Post a Comment